Pilot Studies
The Otricath was tested in a series of pilot studies at the Texas Heart Institute to determine the hepatic venous catheterization techniques, infusion and withdrawal protocols, and catheter design iterations. We performed in-and-out drug infusions simultaneously with a combination of doxorubicin and etoposide.
Critical study criteria were to establish time-on-target at the local hepatic site while withdrawing waste (to protect the heart tissue) and to compare drug concentrations in tissue, waste, and serum.
We established a veno-venous (V-V) steady-state, closed loop circuit confirmed by fluoroscopy (Figure 1); the drug regimen was then administered to the site. The schedule for sample collection was designed to estimate and determine, by trial-and-error, the time of occurrence and the duration of drug exposure.
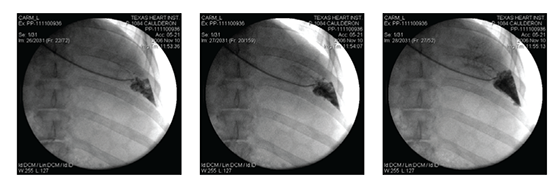
Figure 1 A catheter positioned in the hepatic vein; time sequence of the radio-opaque dye injected into the targeted area.
Serum samples were taken from the withdrawal syringe, right ventricle, aorta, and femoral vein at 10-minute intervals during and 30-minute intervals after infusion. Yellow tissue-marking dye was infused to mark the distribution of the 30-minute infusion (Figure 2). Blood serum samples and frozen tissue sections from the target liver site, waste, heart, and distal liver sites were analyzed with high-pressure liquid chromatography (HPLC).
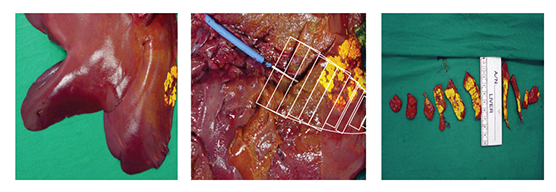
Figure 2 Yellow tissue-marking dye showing the targeted area and the tissue-sampling demarcation.
Tissue-Sampling Protocol
Figure 3 shows the sample mapping key for hepatic tissue harvest and analysis of drug concentrations. A total of 10 sites were sampled. Tissue was immediately frozen for subsequent HPLC to determine drug concentrations. High concentrations of doxorubicin were found in local targeted hepatic tissue (Figure 4).
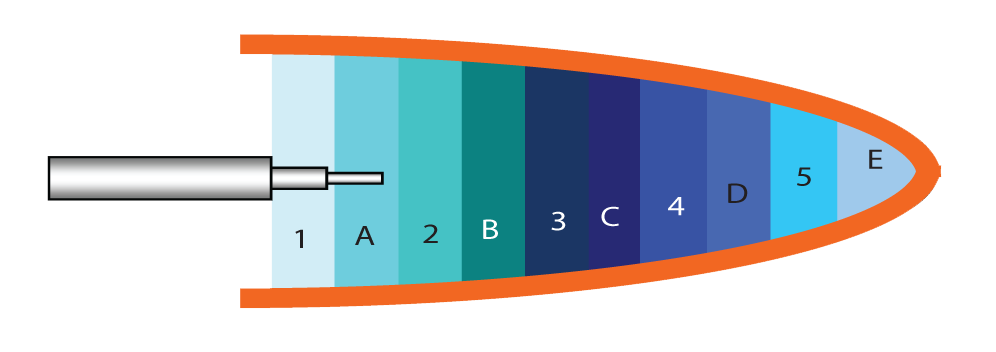
Figure 3 Tissue sampling key
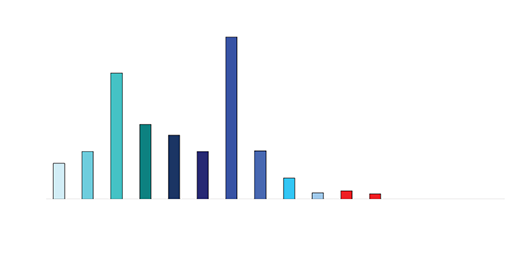
Figure 4 Doxorubicin concentrations in hepatic tissue
Results
Serum and tissue samples were obtained from the local hepatic tissue site, the waste syringe, heart tissue, the systemic circulation, and distal normal liver sites.
Drug levels in the serum waste were 10 times higher than in the tissue (0.3-0.4mcg/mL). Negligible levels were found in the cardiac tissue, untreated hepatic tissue, and systemic blood serum (Figures 4 and 5). A comparative analysis between systemic IV injection and sequences of local venous drug delivery via the Otricath is shown in (Figure 7).
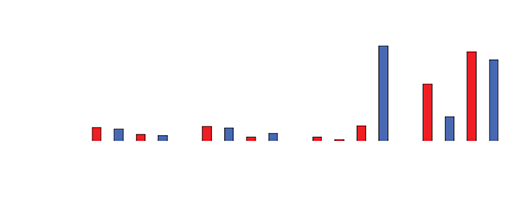
Figure 5
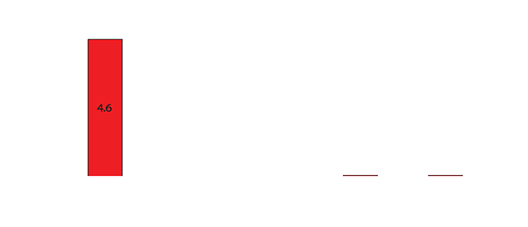
Figure 6
Conclusions
The Otricath offers considerable control over blood flow in the venous hepatic circulation, enabling localized delivery and distribution of chemotherapeutic agents and potentially minimizing chemotherapy-related toxicity. Further studies are ongoing to determine the appropriate dosing schedules and to define appropriate time-on-target requirements

NHC, LLC | 2450 Holcombe Blvd., Suite X | Houston, TX 77021
713.839.8600 | info@otricath.com
The Device
Pilot Studies
The Procedure
Advantages
Milestones

